Your cart is currently empty!
Study of Cysteine Residues
Enlightening fundamental and applied research.
Cysteine is an amino acid that is very interesting from a biological point of view. Their thiol side chain is indeed implicated in many enzymatic reactions and can form a disulfide bridge with another cysteine residue in the same protein or in another one.
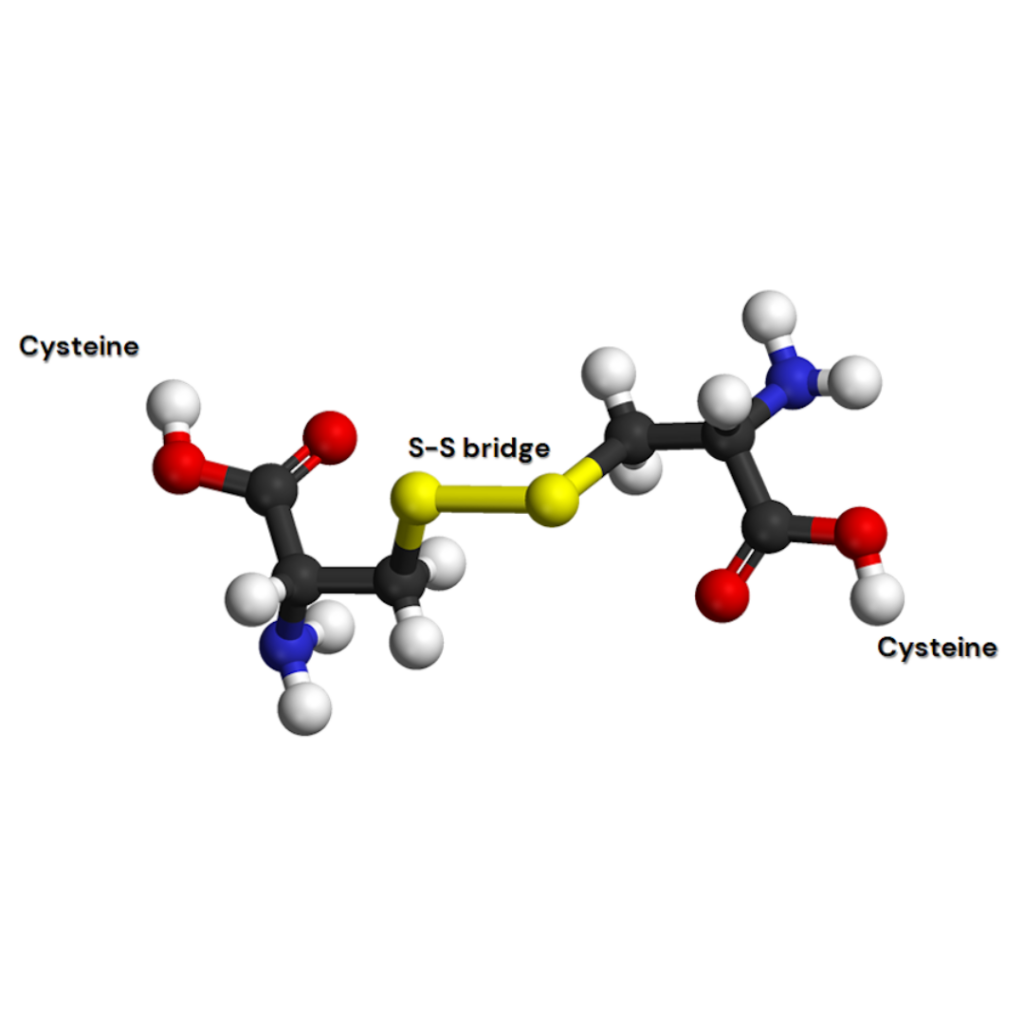
The workflow for studying cysteine residues
There is a variety of tools and strategies available to study cysteine using mass spectrometry. Our team works with you to customize and adapt the best workflow to meet your goals. The following are some examples:
- Differential alkylation (to compare free cysteine between two conditions)
- Disulfide bridge mapping (using native dipeptides)
- Targeted quantification
- Comparative proteomics
- Intact protein analyses
Allumiqs Omics Capabilities
Proteomics
Get quantitative data for up to 6500 proteins in your samples with our label-free quantitative proteomics workflow.
Metabolomics
Sample profiling using either untargeted metabolomics or selected panels of targeted metabolites is the best technique.
Lipidomics
Get in-depth profiling of many classes of biologically relevant lipids using our high resolution instruments.
Data Analytics
We help customers unlock the value and potential of their data with clearer, deeper insights.
Let’s connect
Get in touch with our experts
