Your cart is currently empty!
N-Terminal Protein Sequencing
Chemistry hand in hand with mass spec.
Using a combination of chemical modifications, enrichment methods, and mass spectrometry, we can produce a map of the “N-Terminome” of a sample.

Allumiqs Capabilities
Why study the N-Terminal of a protein?
Many precursor proteins must undergo post-translational modifications to become biologically active. For example, the majority of peptide hormones are produced as pro-hormones that need to be proteolytically cleaved for hormonal activity. Therefore, workflows that enable the identification of the N-Terminal of one or more proteins can become very interesting. Using a combination of chemical modifications, enrichment methods, and mass spectrometry, we can produce a map of the “N-Terminome” of a sample.
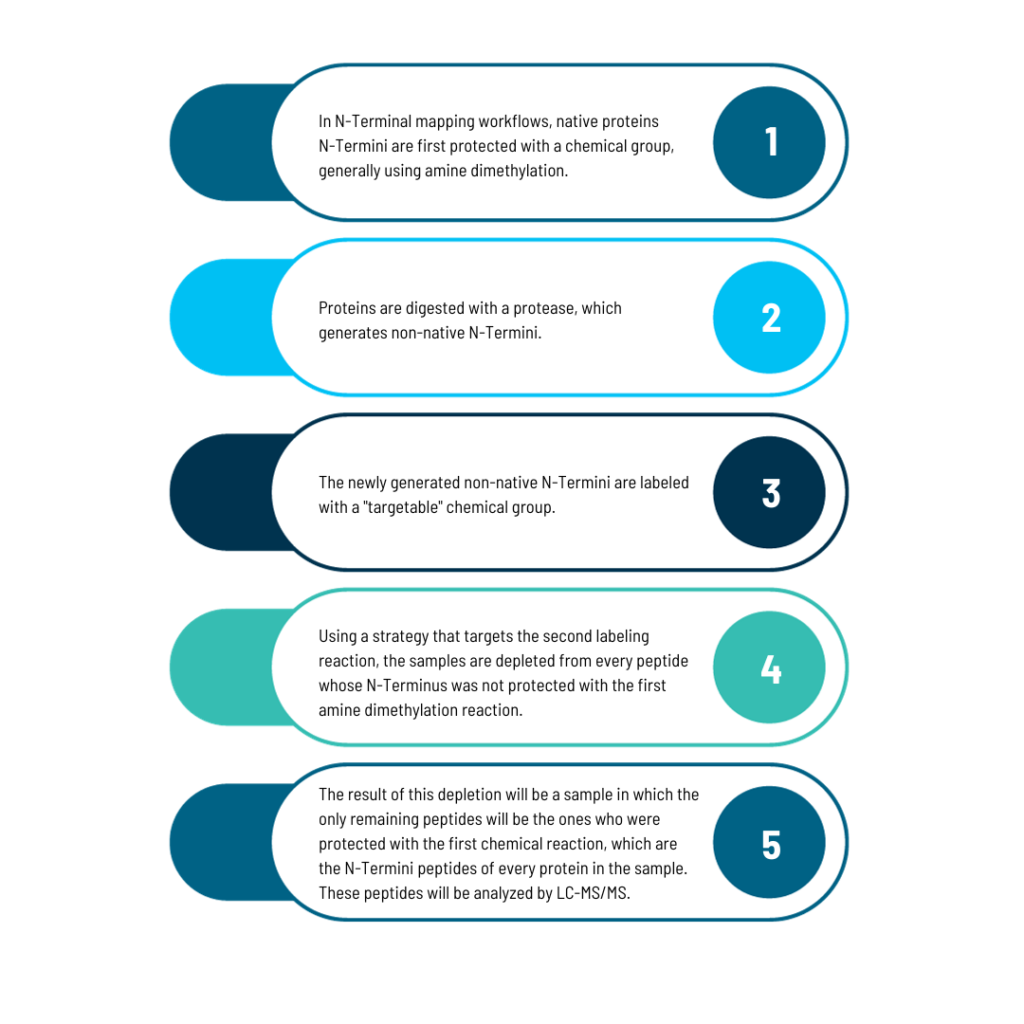
N-Terminal Mapping
The workflow for studying the N-Terminus of proteins
The workflow outlined here can be used to study the N-Terminus of pure proteins or to identify variations in the N-Termini of every detectable protein in a sample.
Our Omics Capabilities
Multiomics
Get the big picture by combining proteomics, lipidomics, and metabolomics data.
Lipidomics
Get in-depth profiling of many classes of biologically relevant lipids using our high resolution instruments.
Metabolomics
Sample profiling using either untargeted metabolomics or selected panels of targeted metabolites is the best technique.
Data Analytics
We help customers unlock the value and potential of their data with clearer, deeper insights.
Let’s connect
Get in touch with our experts
